Chemical properties
The chemical side of a soil is extremely important of course and is about the correct balance of the available nutrients in the soil. This is largely determined by the organic-matter content and its humus percentage; this is the 'pantry' of nutrients on any farm. The extent to which minerals have a dominant presence or not, affects the release of specific nutrients. Supplementing shortages is important, but the right balance is even more important. The soil only produces nutrients if you have the right balance.
Agricultural chemistry committed an error by designating the most common elements as the most important. Fertilisers are a result of SSP thinking ** which is focused on just three elements, when a crop requires more than 40 elements to be able to flourish.
A balanced soil chemistry
It starts with the minerals balance in the soil. The task of the fertilisers is to balance the chemistry of the soil and to feed the crop. The ideal content of the clay-humus complex (CEC) is 60-70% calcium, 10-20% magnesium and 2-5% potassium, which always leaves 10% that can be filled with hydrogen. Hydrogen determines the pH of the soil and is crucial to keeping soil life active and creating availability of the nutrients.

The chemical soil fertility is largely determined by the presence of macro-elements, the presence of micro-elements or trace elements, the acidity or pH, the salt content or EC and the cation-exchange capacity or CEC.
Macro-elements
Macro-elements are the most important nutrients for plants. They occur naturally in the soil to some extent and can be supplemented with fertilisers, manure and compost. Legumes are able to bind nitrogen. The macro-elements are calcium (Ca), magnesium (Mg), potassium (K), sulphur (S), phosphate (P), nitrogen (N), sodium (Na).
Micro-elements
Trace elements are nutrients that plants need in small doses. The trace elements are boron (B), copper (Cu), manganese (Mn), cobalt (Co), silicon (Si), zinc (Zn), iron (Fe) and molybdenum (Mo). Signs of shortage occur when the disappearance of trace elements through the crops is not compensated adequately with supplements by means of fertiliser, manure or compost, or when the availability of certain elements is limited by the pH or mineral imbalance in the soil. It is difficult to solve a lack of trace elements in the plant at source, as many shortages are the result of a shortage or surplus of another mineral in the soil. Crop analyses can help to trace an acute shortage and to solve it at plant level. Soil analyses provide insight into the total mineral balance which tells you where shortages can be expected, which can then be solved.
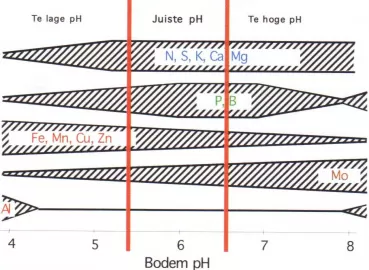
Acidity (pH)
Acidity (pH) is a measure of the concentration of free hydrogen ions (H+). A high concentration in the soil signifies a low pH, whilst a low concentration equals a high pH. Soils and other substances with a pH below 6 are called acidic, whilst those with a pH above 6 are known as base or alkaline.
The form in which macro-elements and micro-elements occur in the soil depends on the pH. This means that the pH exercises a significant influence on the availability of nutrients for plants, as plants cannot absorb macro-elements and micro-elements in all their forms. A pH that is less than 4.5 restricts the availability of a number of elements. Soil-life activity and consequently the mineralisation of organic matter are limited in that situation.
Agricultural soils are often limed in order to keep the pH sufficiently high. Adding lime also stimulates soil life and promotes the breakdown or organic matter.
Salt levels
The salt level is the sum of all the mineral salts that are present in the soil. They can originate from the soil itself, fertiliser, organic manure and in coastal areas from salt marshes or tidal marshes. When the salt levels in the soil are higher than in the cells of the plant roots, the moisture is drawn from the roots and the fine hair roots die off. Over time this impedes the moisture and nutrients absorption by the plant and causes reduced growth or death of the plant. Crops vary in the extent to which they tolerate salt and the salt levels at which they can still provide a good yield. Some crops grow well in salty soils.
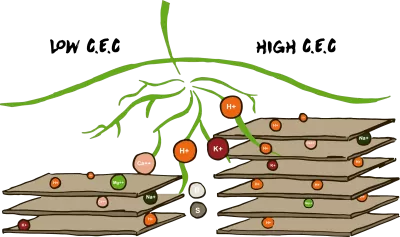
CEC
CEC is the abbreviation of cation exchange capacity, which notes the amount of cations can be bound to the soil. These cations are mainly Ca2+, Mg2+, K+, Na+ and to a lesser extent NH4+ , Al3+, Fe2+, Mn2+ and H+. Plenty of calcium (Ca2+) in the adsorption complex is important to the soil structure. Little calcium or lots of potassium or magnesium bound to clay provides a less good structure. Calcium keeps the clay platelets at a sufficient distance from each other and in doing so produces an airy structure. If there is a lot of potassium or magnesium between the clay platelets, the clay platelets come too close together and the soil is harder to work.
The balance between Ca and Mg is therefore crucial to the right structure of a soil, as it determines the amount of room there is for water and oxygen and that in turns determines the extent of the soil biology.